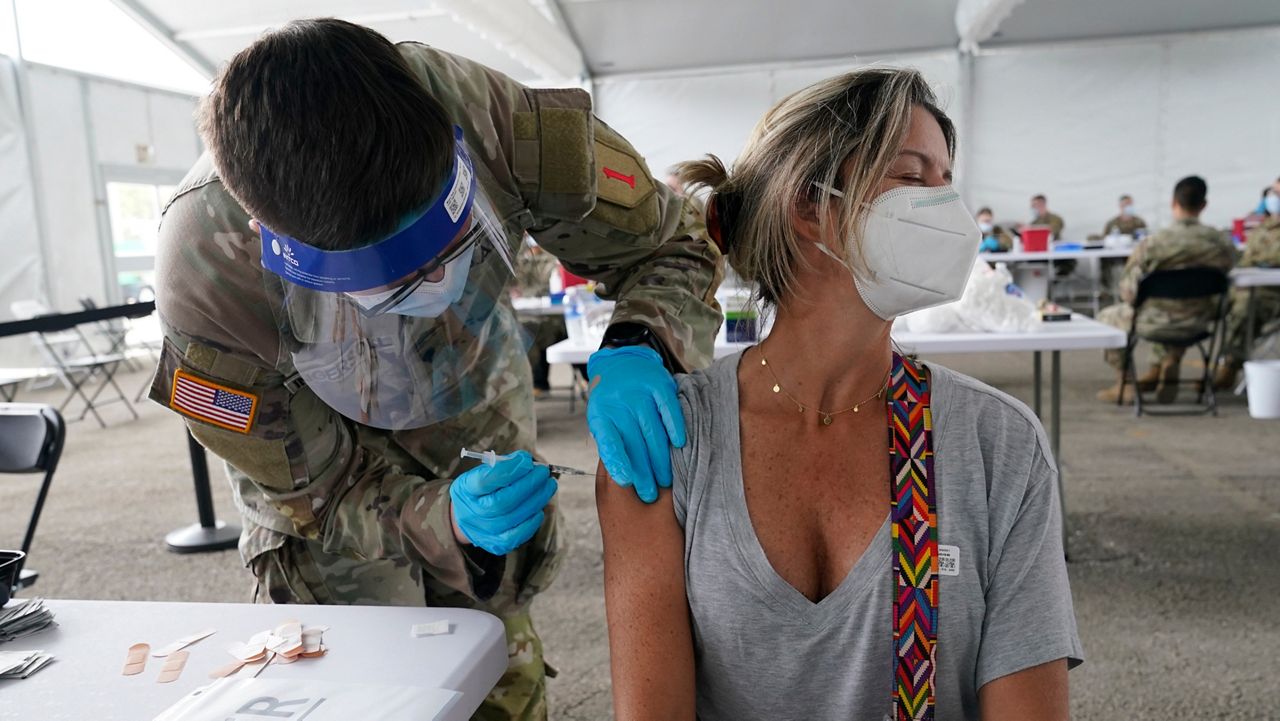
According to a new Centers for Disease Control and Prevention (CDC) study, the Pfizer-BioNTech and Moderna COVID-19 vaccines are 90% effective under "real-world" conditions, a promising sign for the vaccines' efficacy against COVID-19 moving forward.
The study, which was published in the CDC's Morbidity and Mortality Weekly Review on Monday, also suggested that a single dose of either vaccine is 80% effective in preventing coronavirus infections.
"These findings indicate that authorized mRNA COVID-19 vaccines are effective for preventing SARS-CoV-2 infection, regardless of symptom status, among working-age adults in real-world conditions," the CDC said in the study. "COVID-19 vaccination is recommended for all eligible persons."
The study tested 4,000 health care workers, first responders, and other frontline and essential workers in eight locations across six states – Arizona, Florida, Minnesota, Oregon, Texas, and Utah. About 63% of participants received Pfizer, 30% received Moderna, and 5 people received Johnson & Johnson.
The study was conducted from December to March, with most receiving doses in the last two weeks of December.
"This study shows that our national vaccination efforts are working," CDC Director Dr. Rochelle Walensky said in a statement. "The authorized mRNA COVID-19 vaccines provided early, substantial real-world protection against infection for our nation’s health care personnel, first responders, and other frontline essential workers."
"These findings should offer hope to the millions of Americans receiving COVID-19 vaccines each day and to those who will have the opportunity to roll up their sleeves and get vaccinated in the weeks ahead," she added. "The authorized vaccines are the key tool that will help bring an end to this devastating pandemic."
Among those fully vaccinated (2,479), only three had confirmed COVID-19 infections; in those partially vaccinated (477), eight cases were reported. Among the 944 who were not vaccinated, 161 had confirmed cases.
No deaths were reported in the study.